- Electromagnetism
- /
- Electrostatic
- /
- Coulomb's Law
Electric charges repel or attract each other with a force that decreases rapidly with the distance between them. Aside from the atomic scale, the molecules, solids, liquids, gases and all things involving the interaction of man with the environment, the elemental forces involved are gravity and the electromagnetic force. Frictional forces, chemical bonds, viscosity and many of the forces that make the gears turn in industries are manifestations of forces between charges. Here we shall study the forces between static charges, known as electrostatic force, which is described by Coulomb's Law.
Gravitational forces are dominant on a cosmic scale.
The electric forces are dominant in scales above the atomic one.
Coulomb's Law
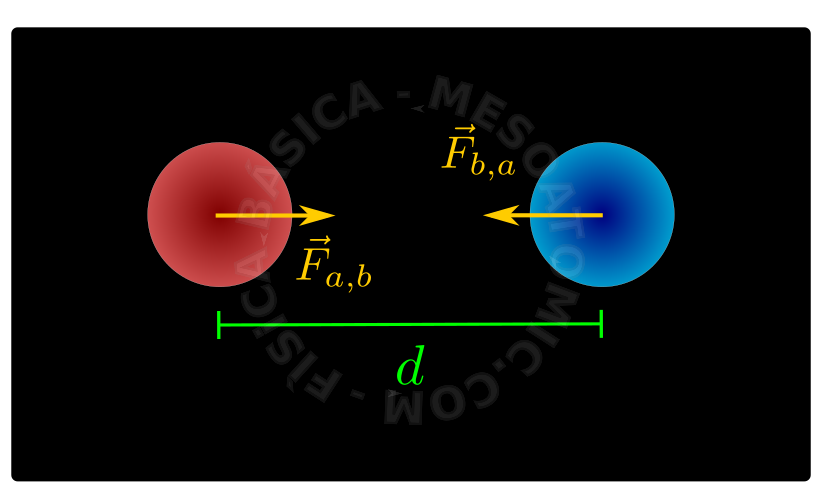
Interaction Between Point Charges
The electric interaction force between two point charges has the following properties:
- It is directly proportional to the product of the two electric charges.
- It is inversely proportional to the square of the distance between the two electric charges.
- It depends on the environment where the charges are immersed (permittivity of the medium). The higher the permittivity, the smaller is the force between charges.
- It is oriented along a straight line connecting the two charges.
- The forces between charges are a couple of action and reaction, i.e., satisfy Newton's third Law (law of action and reaction).
- Electrostatic Constant of the Medium\((k)\)
- The force between two charges is maximum in the void. The surrounding material changes the permittivity of the medium. If electric charges are dipped in a material medium, the value of forces between them becomes smaller, proportionally to \(k\).
Principle of Superposition
When two or more charges exert simultaneous forces on a given charge, it is observed that the total force on the latter is the vector sum of the forces that each charge exerts individually.
